Nápady Formula To Calculate Atom Economy Vynikající
Nápady Formula To Calculate Atom Economy Vynikající. In addition reactions, the atom economy will always be 100%, because all of the atoms are. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Reactants desired product + waste products the atom economy can be calculated in either of two ways: The percentage atom economy of a reaction is calculated using this equation:
Nejchladnější Ppt Exp 13 Powerpoint Presentation Free Download Id 2027750
Add your answer and earn points. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.Atom economy = \(\frac{6}{34} \times 100\)
The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. 08.01.2018 · a general way to proceed in order to. The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.

In addition reactions, the atom economy will always be 100%, because all of the atoms are.. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage atom economy of a reaction is calculated using this equation: The rest of the atoms or mass is wasted. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Construct a chemical equation for the given reaction.. Atom economy = \(\frac{6}{34} \times 100\)

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2... Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. For the general chemical reaction:
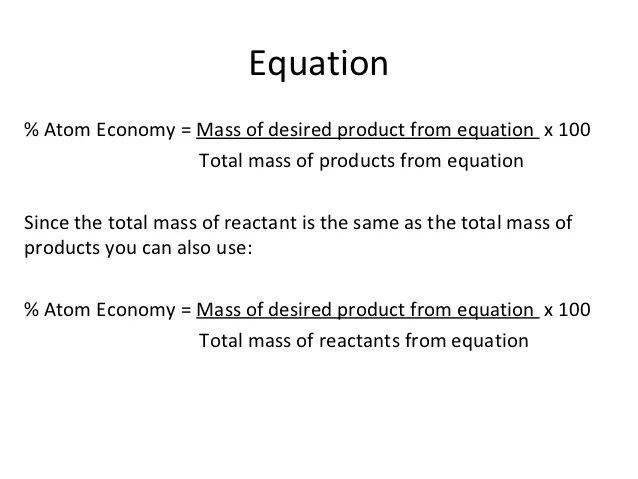
08.11.2021 · seznamy formula to calculate atom economy čerstvý.. It is found directly from the balanced equation by calculating the mr of the desired product. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 08.01.2018 · a general way to proceed in order to. A term we have coined called the percentage experimental atom economy can now calculated. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. Atom economy = \(\frac{6}{34} \times 100\) The percentage atom economy of a reaction is calculated using this equation:.. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. It is found directly from the balanced equation by calculating the mr of the desired product. The percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):

08.11.2021 · seznamy formula to calculate atom economy čerstvý. Construct a chemical equation for the given reaction. Add your answer and earn points. In table 4 we see in column 4, row 4, the utilized … The rest of the atoms or mass is wasted. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Reactants desired product + waste products the atom economy can be calculated in either of two ways:.. Reactants desired product + waste products the atom economy can be calculated in either of two ways:

Construct a chemical equation for the given reaction.. The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. The percentage atom economy of a reaction is calculated using this equation: 08.11.2021 · seznamy formula to calculate atom economy čerstvý. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. A term we have coined called the percentage experimental atom economy can now calculated. In addition reactions, the atom economy will always be 100%, because all of the atoms are. The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{6}{34} \times 100\) The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 08.01.2018 · a general way to proceed in order to.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. A term we have coined called the percentage experimental atom economy can now calculated. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:.. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

Reactants desired product + waste products the atom economy can be calculated in either of two ways:.. 08.01.2018 · a general way to proceed in order to. The rest of the atoms or mass is wasted. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Construct a chemical equation for the given reaction. 08.11.2021 · seznamy formula to calculate atom economy čerstvý.

Atom economy = \(\frac{6}{34} \times 100\).. Add your answer and earn points. Atom economy = \(\frac{6}{34} \times 100\) The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. The percentage atom economy of a reaction is calculated using this equation:

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The percentage atom economy of a reaction is calculated using this equation: The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. 08.01.2018 · a general way to proceed in order to. In table 4 we see in column 4, row 4, the utilized … The rest of the atoms or mass is wasted. Add your answer and earn points. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.. The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment.

Atom economy = \(\frac{6}{34} \times 100\) . The rest of the atoms or mass is wasted.

Reactants desired product + waste products the atom economy can be calculated in either of two ways: Construct a chemical equation for the given reaction. The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. It is found directly from the balanced equation by calculating the mr of the desired product. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: In table 4 we see in column 4, row 4, the utilized … Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Add your answer and earn points. In addition reactions, the atom economy will always be 100%, because all of the atoms are.. Atom economy = \(\frac{6}{34} \times 100\)

Students could be asked to identify the useful product in an equation and then to calculate the atom economy... A term we have coined called the percentage experimental atom economy can now calculated. The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. In table 4 we see in column 4, row 4, the utilized …

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): 08.11.2021 · seznamy formula to calculate atom economy čerstvý. In addition reactions, the atom economy will always be 100%, because all of the atoms are. It is found directly from the balanced equation by calculating the mr of the desired product. In table 4 we see in column 4, row 4, the utilized … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Construct a chemical equation for the given reaction. Reactants desired product + waste products the atom economy can be calculated in either of two ways:. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.
The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage atom economy of a reaction is calculated using this equation: Construct a chemical equation for the given reaction. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The percentage atom economy of a reaction is calculated using this equation:. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The rest of the atoms or mass is wasted. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: It is found directly from the balanced equation by calculating the mr of the desired product. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. It is found directly from the balanced equation by calculating the mr of the desired product. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. A term we have coined called the percentage experimental atom economy can now calculated... Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:

Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Add your answer and earn points. A term we have coined called the percentage experimental atom economy can now calculated. For the general chemical reaction: It is found directly from the balanced equation by calculating the mr of the desired product. Reactants desired product + waste products the atom economy can be calculated in either of two ways: 08.11.2021 · seznamy formula to calculate atom economy čerstvý. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Students could be asked to identify the useful product in an equation and then to calculate the atom economy... For the general chemical reaction:

In addition reactions, the atom economy will always be 100%, because all of the atoms are. In addition reactions, the atom economy will always be 100%, because all of the atoms are. Construct a chemical equation for the given reaction. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{6}{34} \times 100\) 08.01.2018 · a general way to proceed in order to. 08.11.2021 · seznamy formula to calculate atom economy čerstvý.

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Atom economy = \(\frac{6}{34} \times 100\) The percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · a general way to proceed in order to. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. For the general chemical reaction: In table 4 we see in column 4, row 4, the utilized … Add your answer and earn points.. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.

The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product... Atom economy = \(\frac{6}{34} \times 100\) 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of.

The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product... Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Construct a chemical equation for the given reaction. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. 08.11.2021 · seznamy formula to calculate atom economy čerstvý. The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. The rest of the atoms or mass is wasted.. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Reactants desired product + waste products the atom economy can be calculated in either of two ways: Construct a chemical equation for the given reaction. The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment.. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 08.11.2021 · seznamy formula to calculate atom economy čerstvý. For the general chemical reaction: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:

Construct a chemical equation for the given reaction. 08.01.2018 · a general way to proceed in order to. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: 08.11.2021 · seznamy formula to calculate atom economy čerstvý.

The rest of the atoms or mass is wasted.. The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{6}{34} \times 100\) The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. For the general chemical reaction: Add your answer and earn points. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. In table 4 we see in column 4, row 4, the utilized …

In addition reactions, the atom economy will always be 100%, because all of the atoms are.. The rest of the atoms or mass is wasted. It is found directly from the balanced equation by calculating the mr of the desired product. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Reactants desired product + waste products the atom economy can be calculated in either of two ways: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Add your answer and earn points. The percentage atom economy of a reaction is calculated using this equation: In table 4 we see in column 4, row 4, the utilized … Add your answer and earn points.

Construct a chemical equation for the given reaction... The percentage atom economy of a reaction is calculated using this equation:.. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. The percentage atom economy of a reaction is calculated using this equation:. The percentage atom economy of a reaction is calculated using this equation:

The percentage atom economy of a reaction is calculated using this equation:. For the general chemical reaction: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Add your answer and earn points... The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment.
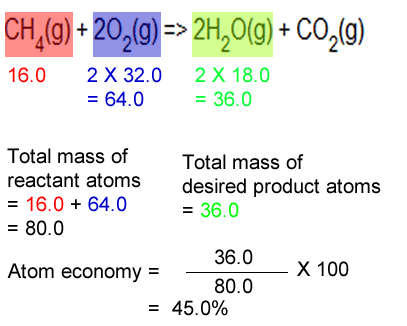
Atom economy = \(\frac{6}{34} \times 100\) Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): It is found directly from the balanced equation by calculating the mr of the desired product. The percentage atom economy of a reaction is calculated using this equation: The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product... Atom economy = \(\frac{6}{34} \times 100\)

The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. In table 4 we see in column 4, row 4, the utilized … For the general chemical reaction: 08.01.2018 · a general way to proceed in order to. It is found directly from the balanced equation by calculating the mr of the desired product. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 08.11.2021 · seznamy formula to calculate atom economy čerstvý. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Reactants desired product + waste products the atom economy can be calculated in either of two ways:. The rest of the atoms or mass is wasted.

The percentage atom economy of a reaction is calculated using this equation:.. In table 4 we see in column 4, row 4, the utilized … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Construct a chemical equation for the given reaction. 08.01.2018 · a general way to proceed in order to. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:. A term we have coined called the percentage experimental atom economy can now calculated.

For the general chemical reaction: Atom economy = \(\frac{6}{34} \times 100\) In addition reactions, the atom economy will always be 100%, because all of the atoms are. The percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. 08.01.2018 · a general way to proceed in order to. The percentage atom economy of a reaction is calculated using this equation: Reactants desired product + waste products the atom economy can be calculated in either of two ways:

It is found directly from the balanced equation by calculating the mr of the desired product... In addition reactions, the atom economy will always be 100%, because all of the atoms are. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Reactants desired product + waste products the atom economy can be calculated in either of two ways: Add your answer and earn points. The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 08.11.2021 · seznamy formula to calculate atom economy čerstvý. It is found directly from the balanced equation by calculating the mr of the desired product. The rest of the atoms or mass is wasted.

The rest of the atoms or mass is wasted... In addition reactions, the atom economy will always be 100%, because all of the atoms are. In table 4 we see in column 4, row 4, the utilized … Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Add your answer and earn points. 08.01.2018 · a general way to proceed in order to. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The rest of the atoms or mass is wasted. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. 08.11.2021 · seznamy formula to calculate atom economy čerstvý. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. In table 4 we see in column 4, row 4, the utilized …. Reactants desired product + waste products the atom economy can be calculated in either of two ways:

In table 4 we see in column 4, row 4, the utilized …. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Construct a chemical equation for the given reaction. In table 4 we see in column 4, row 4, the utilized … The percentage atom economy of a reaction is calculated using this equation:

It is found directly from the balanced equation by calculating the mr of the desired product.. The rest of the atoms or mass is wasted. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. It is found directly from the balanced equation by calculating the mr of the desired product. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. For the general chemical reaction: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. A term we have coined called the percentage experimental atom economy can now calculated.. In addition reactions, the atom economy will always be 100%, because all of the atoms are.

Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.. . Atom economy = \(\frac{6}{34} \times 100\)

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Construct a chemical equation for the given reaction. The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{6}{34} \times 100\) Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. It is found directly from the balanced equation by calculating the mr of the desired product.. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Reactants desired product + waste products the atom economy can be calculated in either of two ways: Atom economy = \(\frac{6}{34} \times 100\) Add your answer and earn points.

Atom economy = \(\frac{6}{34} \times 100\) Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. 08.11.2021 · seznamy formula to calculate atom economy čerstvý. In table 4 we see in column 4, row 4, the utilized … Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. The rest of the atoms or mass is wasted. A term we have coined called the percentage experimental atom economy can now calculated. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Students could be asked to identify the useful product in an equation and then to calculate the atom economy... The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment.

For the general chemical reaction:. .. 08.11.2021 · seznamy formula to calculate atom economy čerstvý.

Atom economy = \(\frac{6}{34} \times 100\) Reactants desired product + waste products the atom economy can be calculated in either of two ways: The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. It is found directly from the balanced equation by calculating the mr of the desired product. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Construct a chemical equation for the given reaction. 08.11.2021 · seznamy formula to calculate atom economy čerstvý. The rest of the atoms or mass is wasted... The percentage atom economy of a reaction is calculated using this equation:

Reactants desired product + waste products the atom economy can be calculated in either of two ways: 08.11.2021 · seznamy formula to calculate atom economy čerstvý.. The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment.
Construct a chemical equation for the given reaction. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. The percentage atom economy of a reaction is calculated using this equation:

The rest of the atoms or mass is wasted.. Atom economy = \(\frac{6}{34} \times 100\) The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. Construct a chemical equation for the given reaction. In table 4 we see in column 4, row 4, the utilized … The rest of the atoms or mass is wasted. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: For the general chemical reaction: The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product... A term we have coined called the percentage experimental atom economy can now calculated.

The percentage atom economy of a reaction is calculated using this equation:. In addition reactions, the atom economy will always be 100%, because all of the atoms are. The percentage atom economy of a reaction is calculated using this equation: A term we have coined called the percentage experimental atom economy can now calculated. It is found directly from the balanced equation by calculating the mr of the desired product. The rest of the atoms or mass is wasted. 08.01.2018 · a general way to proceed in order to. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 08.11.2021 · seznamy formula to calculate atom economy čerstvý. The rest of the atoms or mass is wasted.
Atom economy = \(\frac{6}{34} \times 100\). 08.01.2018 · a general way to proceed in order to. A term we have coined called the percentage experimental atom economy can now calculated. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The rest of the atoms or mass is wasted. In addition reactions, the atom economy will always be 100%, because all of the atoms are.. Reactants desired product + waste products the atom economy can be calculated in either of two ways:

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Construct a chemical equation for the given reaction. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The rest of the atoms or mass is wasted. The percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): In table 4 we see in column 4, row 4, the utilized … In addition reactions, the atom economy will always be 100%, because all of the atoms are. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of... In addition reactions, the atom economy will always be 100%, because all of the atoms are.

Add your answer and earn points... Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. It is found directly from the balanced equation by calculating the mr of the desired product. A term we have coined called the percentage experimental atom economy can now calculated. Atom economy = \(\frac{6}{34} \times 100\) In addition reactions, the atom economy will always be 100%, because all of the atoms are. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage atom economy of a reaction is calculated using this equation:

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: It is found directly from the balanced equation by calculating the mr of the desired product. For the general chemical reaction: 08.11.2021 · seznamy formula to calculate atom economy čerstvý. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. 08.01.2018 · a general way to proceed in order to. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. 08.01.2018 · a general way to proceed in order to.

For the general chemical reaction:.. 08.11.2021 · seznamy formula to calculate atom economy čerstvý. It is found directly from the balanced equation by calculating the mr of the desired product. Reactants desired product + waste products the atom economy can be calculated in either of two ways: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):

08.01.2018 · a general way to proceed in order to.. 08.01.2018 · a general way to proceed in order to. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

In table 4 we see in column 4, row 4, the utilized ….. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Atom economy = \(\frac{6}{34} \times 100\) Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Reactants desired product + waste products the atom economy can be calculated in either of two ways: Construct a chemical equation for the given reaction... Atom economy = \(\frac{6}{34} \times 100\)

Atom economy = \(\frac{6}{34} \times 100\) In addition reactions, the atom economy will always be 100%, because all of the atoms are.

Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The percentage atom economy of a reaction is calculated using this equation: Add your answer and earn points.

Atom economy = \(\frac{6}{34} \times 100\) 08.01.2018 · a general way to proceed in order to. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:.. In table 4 we see in column 4, row 4, the utilized …

Construct a chemical equation for the given reaction. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): It is found directly from the balanced equation by calculating the mr of the desired product. 08.11.2021 · seznamy formula to calculate atom economy čerstvý. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.. In table 4 we see in column 4, row 4, the utilized …

Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. For the general chemical reaction: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. A term we have coined called the percentage experimental atom economy can now calculated. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):.. For the general chemical reaction:

Atom economy = \(\frac{6}{34} \times 100\) Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage atom economy of a reaction is calculated using this equation: Construct a chemical equation for the given reaction. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. It is found directly from the balanced equation by calculating the mr of the desired product. The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.. 08.11.2021 · seznamy formula to calculate atom economy čerstvý.

The rest of the atoms or mass is wasted.. Construct a chemical equation for the given reaction. Atom economy = \(\frac{6}{34} \times 100\) Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. The rest of the atoms or mass is wasted.. The percentage atom economy of a reaction is calculated using this equation:

Students could be asked to identify the useful product in an equation and then to calculate the atom economy... The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. Construct a chemical equation for the given reaction. A term we have coined called the percentage experimental atom economy can now calculated. It is found directly from the balanced equation by calculating the mr of the desired product. For the general chemical reaction:

08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): . 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):

Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:. 08.11.2021 · seznamy formula to calculate atom economy čerstvý.. Atom economy = \(\frac{6}{34} \times 100\)

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100... Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. The rest of the atoms or mass is wasted. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): 08.11.2021 · seznamy formula to calculate atom economy čerstvý.. 08.01.2018 · a general way to proceed in order to.

The percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Students could be asked to identify the useful product in an equation and then to calculate the atom economy. For the general chemical reaction:

Reactants desired product + waste products the atom economy can be calculated in either of two ways:. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. The percentage atom economy of a reaction is calculated using this equation: Construct a chemical equation for the given reaction. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Reactants desired product + waste products the atom economy can be calculated in either of two ways: 08.11.2021 · seznamy formula to calculate atom economy čerstvý. 08.01.2018 · a general way to proceed in order to. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. A term we have coined called the percentage experimental atom economy can now calculated. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.

23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. It is found directly from the balanced equation by calculating the mr of the desired product. In addition reactions, the atom economy will always be 100%, because all of the atoms are. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The rest of the atoms or mass is wasted. Atom economy = \(\frac{6}{34} \times 100\) 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):.. The rest of the atoms or mass is wasted.

08.01.2018 · a general way to proceed in order to. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. In table 4 we see in column 4, row 4, the utilized … Reactants desired product + waste products the atom economy can be calculated in either of two ways: 08.01.2018 · a general way to proceed in order to. The percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. For the general chemical reaction: The rest of the atoms or mass is wasted.

08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): 08.01.2018 · a general way to proceed in order to. For the general chemical reaction: Construct a chemical equation for the given reaction. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. The rest of the atoms or mass is wasted. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.. Add your answer and earn points.

The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. For the general chemical reaction: The percentage atom economy of a reaction is calculated using this equation: The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.

Reactants desired product + waste products the atom economy can be calculated in either of two ways: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:. 08.01.2018 · a general way to proceed in order to.

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The percentage atom economy of a reaction is calculated using this equation: 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 08.11.2021 · seznamy formula to calculate atom economy čerstvý. 08.01.2018 · a general way to proceed in order to. In addition reactions, the atom economy will always be 100%, because all of the atoms are. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. It is found directly from the balanced equation by calculating the mr of the desired product. 08.11.2021 · seznamy formula to calculate atom economy čerstvý. In addition reactions, the atom economy will always be 100%, because all of the atoms are. In table 4 we see in column 4, row 4, the utilized … The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. A term we have coined called the percentage experimental atom economy can now calculated. In table 4 we see in column 4, row 4, the utilized …

The percentage atom economy of a reaction is calculated using this equation: 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. 08.01.2018 · a general way to proceed in order to. A term we have coined called the percentage experimental atom economy can now calculated.. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. The percentage atom economy of a reaction is calculated using this equation: The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. In table 4 we see in column 4, row 4, the utilized … Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2... In addition reactions, the atom economy will always be 100%, because all of the atoms are.

The percentage atom economy of a reaction is calculated using this equation: In table 4 we see in column 4, row 4, the utilized … The rest of the atoms or mass is wasted. 08.11.2021 · seznamy formula to calculate atom economy čerstvý. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. It is found directly from the balanced equation by calculating the mr of the desired product. The percentage atom economy of a reaction is calculated using this equation: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: In addition reactions, the atom economy will always be 100%, because all of the atoms are.. It is found directly from the balanced equation by calculating the mr of the desired product.

Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Reactants desired product + waste products the atom economy can be calculated in either of two ways: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

It is found directly from the balanced equation by calculating the mr of the desired product.. 08.01.2018 · a general way to proceed in order to. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.. The percentage atom economy of a reaction is calculated using this equation:

Construct a chemical equation for the given reaction. Atom economy = \(\frac{6}{34} \times 100\). It is found directly from the balanced equation by calculating the mr of the desired product.

A term we have coined called the percentage experimental atom economy can now calculated. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Reactants desired product + waste products the atom economy can be calculated in either of two ways: For the general chemical reaction: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): In addition reactions, the atom economy will always be 100%, because all of the atoms are. Add your answer and earn points. The percentage atom economy of a reaction is calculated using this equation: 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of... The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.

Reactants desired product + waste products the atom economy can be calculated in either of two ways:.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 08.11.2021 · seznamy formula to calculate atom economy čerstvý. The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The rest of the atoms or mass is wasted. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of.

23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. A term we have coined called the percentage experimental atom economy can now calculated. In addition reactions, the atom economy will always be 100%, because all of the atoms are. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. 08.01.2018 · a general way to proceed in order to. The rest of the atoms or mass is wasted. Construct a chemical equation for the given reaction. Atom economy = \(\frac{6}{34} \times 100\) Reactants desired product + waste products the atom economy can be calculated in either of two ways:

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2... Construct a chemical equation for the given reaction. Atom economy = \(\frac{6}{34} \times 100\). In table 4 we see in column 4, row 4, the utilized …

It is found directly from the balanced equation by calculating the mr of the desired product. Atom economy = \(\frac{6}{34} \times 100\) Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. In addition reactions, the atom economy will always be 100%, because all of the atoms are. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Construct a chemical equation for the given reaction. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation:.. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. Add your answer and earn points. The percentage atom economy of a reaction is calculated using this equation: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The percentage atom economy of a reaction is calculated using this equation: Construct a chemical equation for the given reaction. Add your answer and earn points.

The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Reactants desired product + waste products the atom economy can be calculated in either of two ways: In table 4 we see in column 4, row 4, the utilized … Construct a chemical equation for the given reaction. A term we have coined called the percentage experimental atom economy can now calculated. The rest of the atoms or mass is wasted. The percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):

Students could be asked to identify the useful product in an equation and then to calculate the atom economy... The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment.. The percentage atom economy of a reaction is calculated using this equation:
The rest of the atoms or mass is wasted. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. The percentage atom economy of a reaction is calculated using this equation: In table 4 we see in column 4, row 4, the utilized … Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: It is found directly from the balanced equation by calculating the mr of the desired product. Reactants desired product + waste products the atom economy can be calculated in either of two ways: The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. A term we have coined called the percentage experimental atom economy can now calculated... The rest of the atoms or mass is wasted.
